Look UP! The Research
Air is how we refer to the Earth’s atmosphere. The atmosphere is the layer of gases surrounding the earth. We can split this layer into 5 sections: the troposphere, the stratosphere, the mesosphere, the thermosphere, and the exosphere. The troposphere is the layer of gases closest to the surface of the Earth and is the air we breathe – this is where most of our research is focussed!
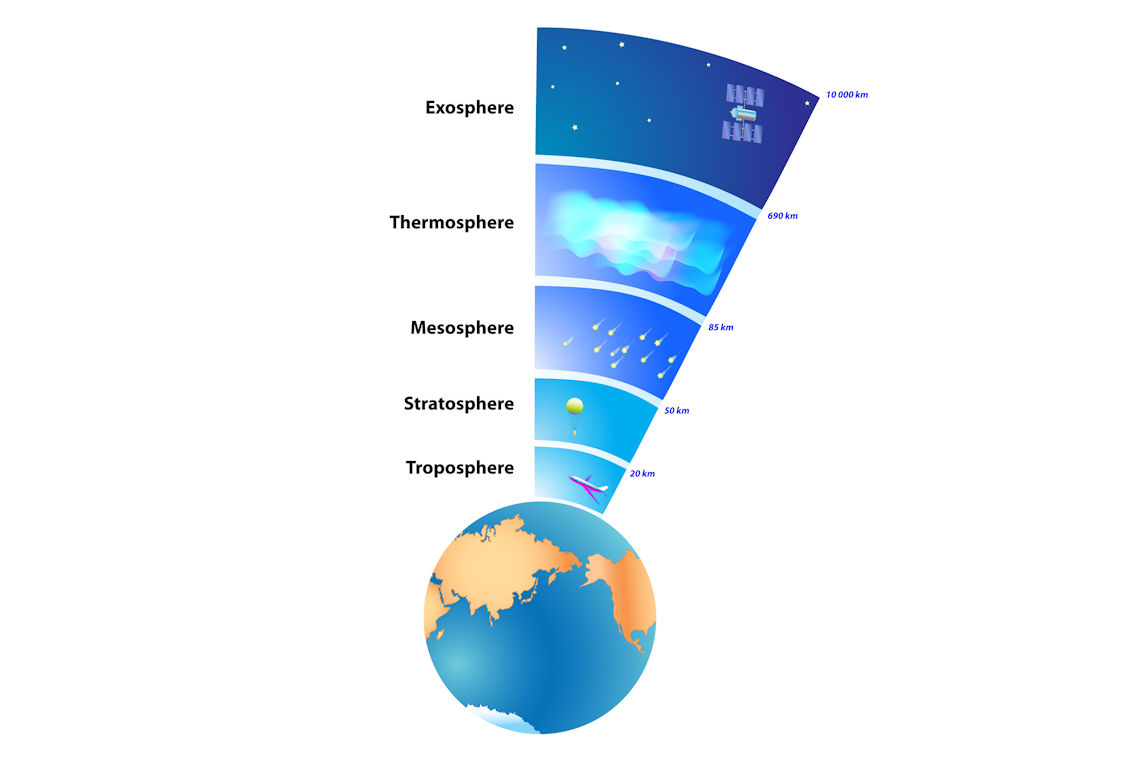
(© Edesignua)
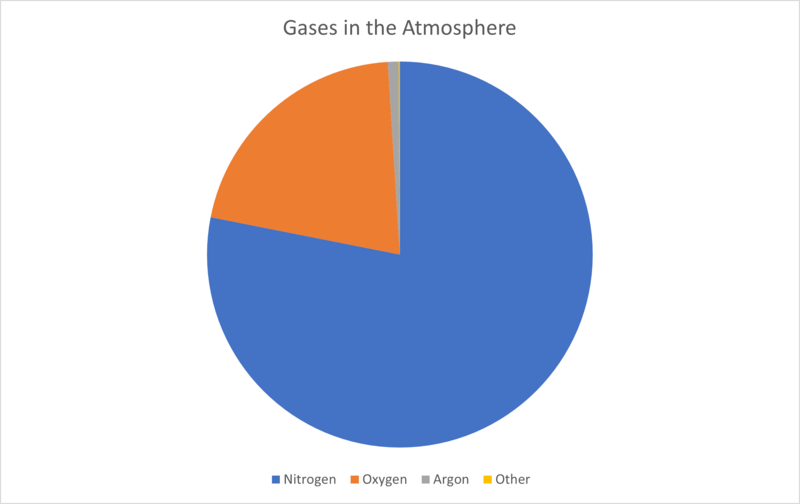
The atmosphere is made up of mainly nitrogen (N2 - 78.1%) with some oxygen (O2 - 20.9%) and small amounts of argon (Ar - 0.9%). The remaining 0.1% is made up of other gases like carbon dioxide (CO2), water vapour (H2O) and ozone (O3). There are also tiny particles of liquids or solids (called aerosols) in the atmosphere. These can be natural or man-made.
Sunlight plays a very important role in the chemistry of the atmosphere through photolytic reactions. Photolytic reactions or photolysis is when high energy sunlight provides enough energy for a reaction to occur that wouldn’t occur otherwise. For example, sunlight may cause oxygen (O2) to break into two Oxygen atoms (O). These atoms are very reactive and can react with other gases in the atmosphere including another O2 molecule (this is how ozone is formed!).
O2 + sunlight à O + O
O + O2 à O3
Oxygen atoms (O) can also react with water (H2O) to generate OH radicals – these are very important as they help to remove compounds from the atmosphere. In fact, they are often referred to as the ‘detergent of the atmosphere’ as they help to keep it clean!
O + H2O à OH + OH
As this reaction requires sunlight, OH radicals are only produced in the daytime, and more are produced the sunnier it is. This means that the atmosphere is generally cleaner during the day and even more so when there is sunny weather!
There are lots of different types of pollution – can you name a few? The pictures below show a few examples.
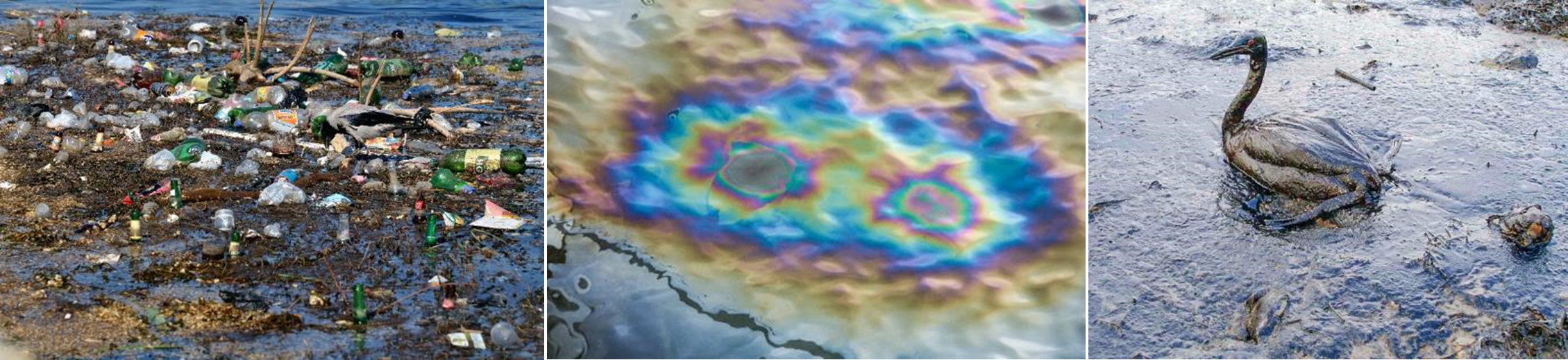
In our research, we focus on air pollution. Air pollution is defined as the contamination of the air by particles and gases that can be harmful to human health and the environment.
Air pollution can be caused by human activity (e.g. emissions from factories or cars) or natural processes (e.g. forest fires or volcanic eruptions).

Air pollution caused by human activity is much easier for us to control and so it is the focus of most of our research.
Do you recognise the event shown in the picture below?

The image shows ‘smog’. The word ‘smog’ was first used in the 1900’s and, as a combination of ‘smoke’ and ‘fog’, was used to differentiate between natural mist/fog and that created by excessive air pollution in the atmosphere. These days, factories burn less solid fuel (like coal) and so generate less smoke. However, we still get smog – why is this? Today, the smog we see is known as photochemical smog which is produced when sunlight reacts with pollutants in the atmosphere (remember the photolytic reactions we talked about in the last section?)
Smog is usually associated with poor air quality in places like China where frequent smogs mean people may wear masks to protect themselves or even stay inside on particularly bad days to avoid the air pollution. However, it’s important to remember that we also see significant smog events in places like London and Krakow (a major city in Poland).

Smog is a useful example of air pollution as we can see it however, most impacts of air pollution are not visible in our everyday life as lots of these harmful gases are colourless and odourless. There are many other more subtle effects of air pollution including:
-
Global warming
-
Ice melt
-
Costal erosion
-
Extreme weather events (like hurricanes or heatwaves)
-
Breathing problems and other illnesses
To protect us and the environment. Some chemicals in the air have limits imposed by governments, this means that the amount of this chemical in the atmosphere must remian below a certain level. As a result, these chemicals need to be monitored accurately to see whether levels of pollutants remain below the limits set. We can also look at other important things like:
-
The relationship between pollutants (i.e. if one increases, how does that effect the levels of another?)
-
The realtionship between pollutants and health impacts for humans and animals
-
The relationship between pollutants and biodiversity
For example,
Scientists measure many types of air pollutants at sites around the UK. Running an air quality network (https://uk-air.defra.gov.uk/) is expensive and so there are a limited number of sites around the UK that The UK Department for Environment, Food and Rural Affairs (Defra) can site science instruments to measure the levels of pollutants in the air.
The UK Air Quality Network focuses on primary pollutants that are produced from human activity:
-
Hydrocarbons (for example, benzene)
-
Nitrogen oxides - nitric oxide (NO) and nitrogen dioxide (NO2)
-
Carbon monoxide (CO)
-
Ozone (O3)
-
Sulfur dioxide (SO2)
-
Particulates i.e. PM2.5 and PM10
There is no use in just measuring the air in one place in the UK every so often! Measurements are taken all over the country in different areas (i.e. cities, towns, countryside) at different time intervals. The video below shows one of the measurement sites used by our research group to study the atmosphere!
We can collect a sample of the air anywhere, not just at sites like these ones. If we collect a sample elsewhere, we have to take it back to the lab and measure the compostition to assess the level of air pollution in that area at that time. Below is a a brief video tour of our lab showing you some of the equipment we use to do this!
Primary Science Teaching Trust (PSTT) - Air Pollution Research Classroom Resources: Air Pollution Research | Primary Science Teaching Trust (pstt.org.uk) (Accessed Jun 2021)
UK AIR - Air Information Resource: Home - Defra, UK
DARE UK - DARE-UK | Detection and Attribution of Regional greenhouse gas Emissions in the UK (bristol.ac.uk), youtube: DARE UK - YouTube

Pollutant - a chemical that can be harmful to humans or the environment
Aerosols - small particles of gas or liquid, mixed in with the air.
Air quality - we use air quality to describe how ‘clean’ the air is. If there is bad air quality, it means there is lots of air pollution
Photolytic reaction - reactions that happen in the air when high-energy sunlight provides enough energy to break compounds into smaller parts
Trend analysis - studying how measurements change over time and looking for patterns in these changes
Peak - when looking at a series of measurements taken at different times, a peak is where the measurements reach a maximum value





