Link UP! The Research
Polymers are large molecules consisting of many ‘repeating units’ of smaller molecules joined together.
Imagine a set of beads on a string:
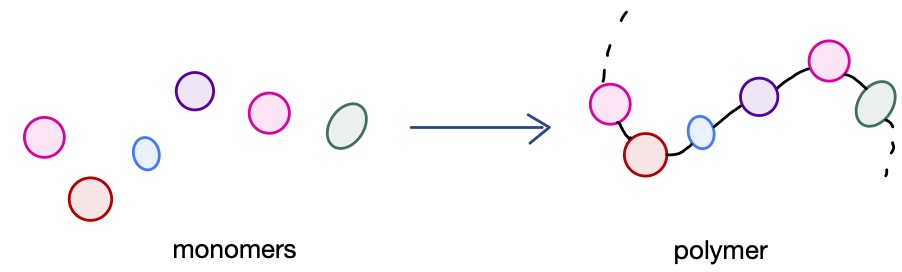
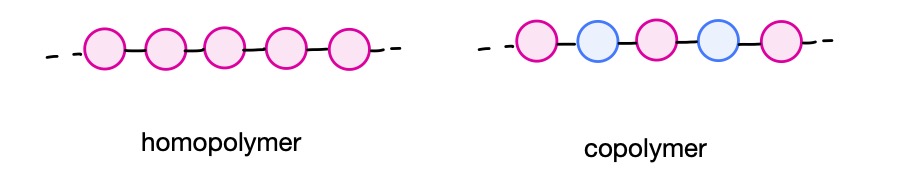
The beads represent the monomer units that are joined together to form the polymer. The monomer units may be identical, producing a homopolymer, or different, producing a copolymer.
Polymers can display lots of useful properties, and are used for a variety of different applications:

Many polymers are found in nature.
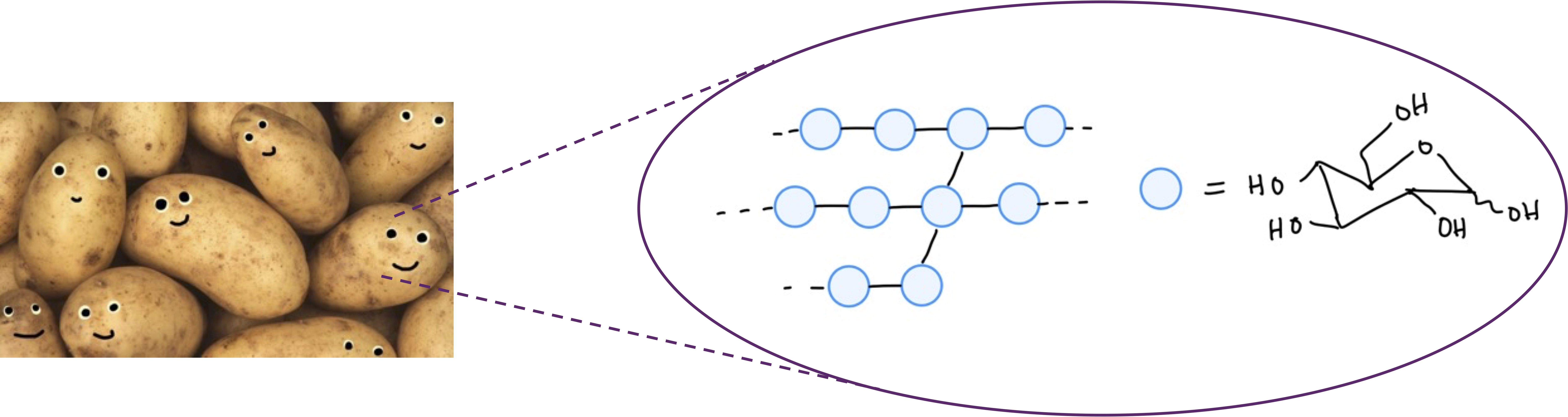
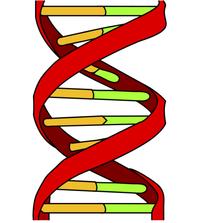
Starch is a polymer of glucose, a type of sugar, which is used for energy storage in plants.DNA is a polymer of nucleic acids. The sequence of monomer units stores information within cells.
DNA is a polymer of nucleic acids. The sequence of monomer units stores information within cells.
The information stored in DNA is used to code for the construction of another type of biological polymer: proteins.
Proteins are polymers of amino acids. The sequence of amino acids in proteins enables them to fold into complex 3D shapes, and fulfil many important biological functions:
Haemoglobin, for example, is used to transport oxygen around the body.
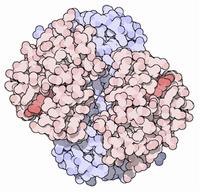
Antibodies are proteins that protect us from disease.
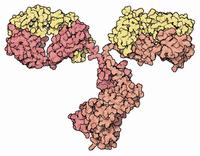
Enzymes make chemical reactions go really quickly!
As chemists, we can design and make polymers. At the moment, we are not as good as natural systems in making polymers with defined sequences, but we are making progress towards that goal!
Many of the polymers that we make fulfil important functions. When designing polymers, we can choose the features we would like the polymer to display.
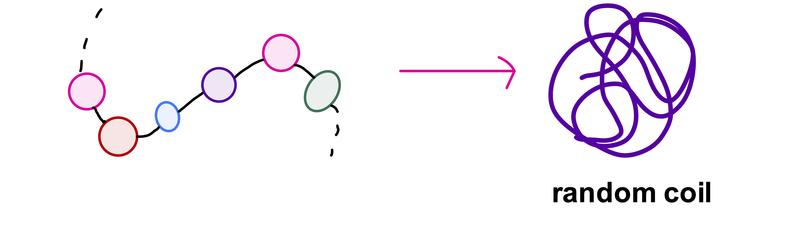
The chain of a polymer can be flexible, allowing it to curl around and adopt different shapes. For example, polymers in solution often resemble a random coil.
Sometimes, chains can interact with each other, forming crosslinked networks:
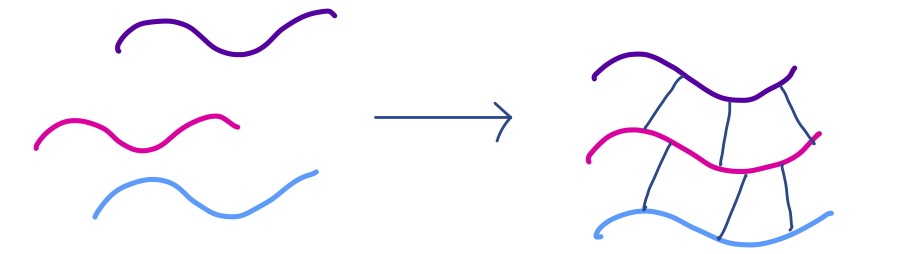
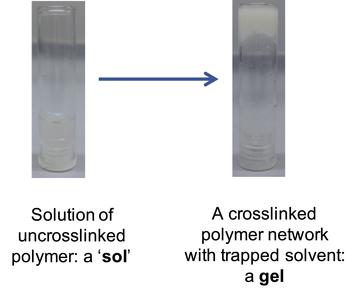
Crosslinking can drastically change the properties of a polymer, often leading to the formation of a film or gel.
Gels are semi-solids that consist of a 3D network of molecules within a liquid. We can consider the liquid to be trapped within the gel network. Often different molecules can be trapped within the gel in this way.
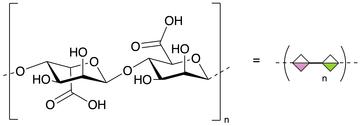
Alginates are polymers of sugars that are produced by some species of algae, and some bacteria.
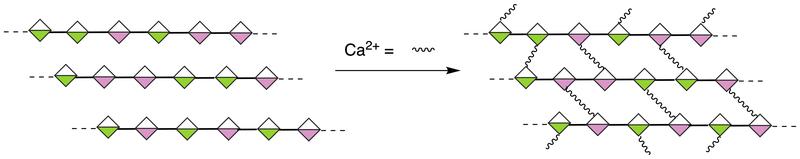
Alginates can form gels in the presence if ions like Ca2+, which form crosslinks between the negatively charged groups on the sugar.
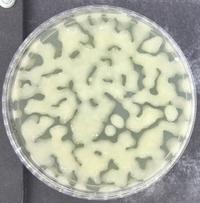
For some bacteria, alginates are a major structural component of biofilms. Biofilms are complex communities of bacteria bound within a network of polymers inlcuding polysaccharides and DNA. The polymer network presents a physical barrier to the delivery of drugs to the site of an infection, making the infection difficult to treat. Biofilms are associated with persistent infections and contribute to the growing problem of antibiotic resistance, where bacteria are no longer affected by the drugs that we rely on to control infections.
A biofilm formed by Pseudomonas aeruginosa:
Challenge: make your own alginate gel!
Polymers can have many uses in healthcare. For example, polymer gels are used in dressings for burns and other wounds. Wound dressings create a sealed environment around the injury, preventing it getting infected, and promote healing by keeping the environment moist.
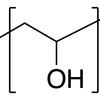
Poly(vinyl alcohol) (PVA) is commonly used to make wound dressings, because it can form a crosslinked gel which is biologically inert.
PVA is also used to make glues. The chemical structure of PVA is:
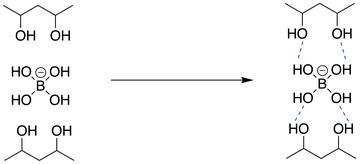
The crosslinks in PVA gels can be formed by the addition of the borate ion [B(OH)4]-. The borate ion can form weak bonds between the OH groups on the polymer chains:
The resulting gels can be loaded with other chemicals that help the healing process, like antibacterial drugs.
The gels are examples of drug delivery systems. Drug delivery systems can make drugs more effective, by increasing the proportion of the drug that reaches the intended site of action.
Drug delivery systems can:
- protect drugs from their surroundings
- make drugs more easily absorbed
- provide control over how the drug is released
In some cases, like a wound dressing, we would like a drug to be released over a sustained period. In other cases we would like the drug to be transported to its site of action, then released all at once, in response to a trigger from the surroundings.
Challenge: make your own model drug delivery system!
What polymers do you see in the world around you?
Have you come across any other gels?

You might find these definitions helpful.
Polymer: large molecule consisting of many repeating units
Monomer: a molecule that forms the basic repeating unit of a polymer.
Gel: a 3D network of molecules within a liquid
Crosslinking: the process of joining two or more polymer chains together
Drug delivery system: a strategy that increases the proportion of a drug that reaches the intended site of action.
Remember that if you have any questions about anything you have read on the research page, you can ask us at one of our live online events!





